Imagine being told that you or your child is going to grow up with a constant weight on their shoulders. A chronic, long term illness, which has no real cure. The treatments are risky, and not nearly effective enough to allow for a person with this diagnosis to lead the kind of life they want. Some of these illnesses are coded into your very DNA, a series of codes at the core of your existence. Illnesses like that have to be impossible to effectively cure, right?
Maybe not.
Vertex Pharmaceuticals and CRISPR Therapeutics have invented a revolutionary type of gene editing technology using something called CRISPR/Cas9. CRISPR stands for “Clustered Regularly Interspaced Short Palindromic Repeats”, and Cas9 is a CRISPR associated enzyme. The technology invented by CRISPR Therapeutics intends to use CRISPR/Cas9 to treat genetic diseases. In particular, the companies have been working on a treatment–if not a potential cure–for sickle cell disease.
Sickle Cell Disease
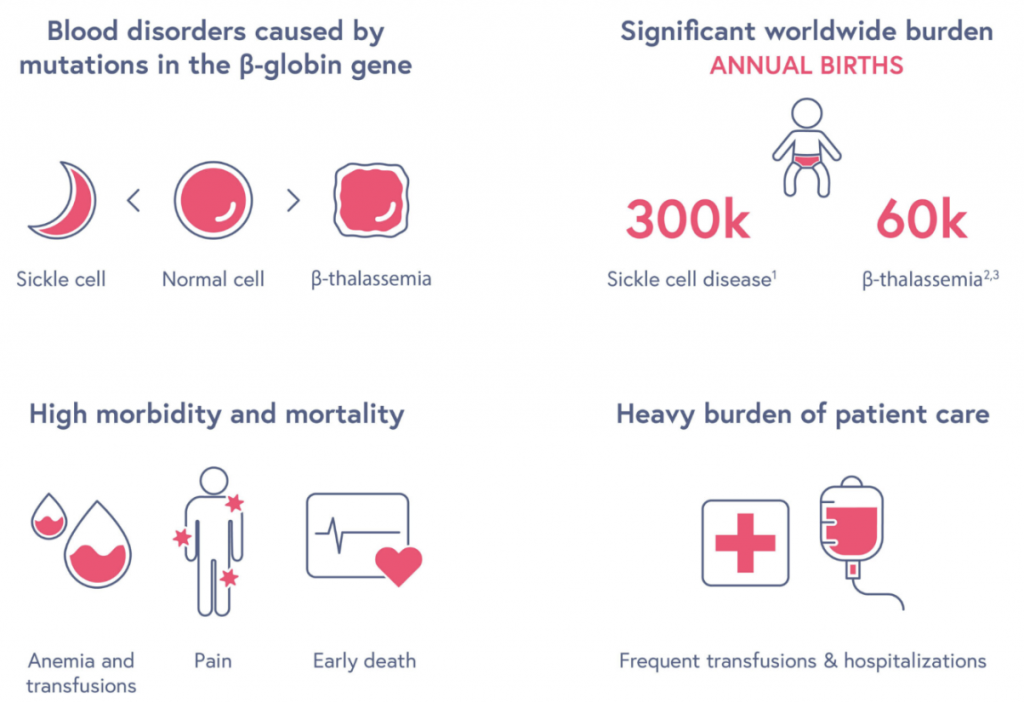
Sickle cell disease is caused by a mutation in the DNA which changes the structure of hemoglobin, an oxygen-carrying protein contained in red blood cells. This mutation causes the red blood cells, normally round, to resemble the c-shaped farming tool called a “sickle”. The hard and sticky sickle cells die much sooner than their healthy counterparts, which results in a constant shortage of red blood cells. Additionally, their odd shape increases the chances of the cells getting stuck in small blood vessels and clogging the blood flow.
The odd shape, alongside the shortage of red blood cells, comes with many serious complications, including pain, infection, acute chest syndrome, anemia and stroke. More often than not, sickle cell disease results in an early death by roughly thirty years.
Complications usually manifest within a person’s first year of life, often at about five months. Thankfully, diagnosis is possible long before the symptoms first appear, usually at newborn screening tests, and the disease can be diagnosed pre-birth. The sooner the diagnosis the better, as treatment needs to begin relatively quickly in order for it to have the most significant effect.
Currently, the only F.D.A.-approved therapy for sickle cell disease is bone marrow or stem cell transplants. Bone marrow is a soft, fatty tissue at the center of your bones in which blood cells are made. Stem cells, on the other hand, are unspecialized cells, meaning they are able to differentiate into any cell and are able to self-renew. The transplant procedures take healthy blood-forming cells from a donor and insert them into the sickle cell patient.
Unfortunately, these transplants are extremely high risk. They also have many serious side effects, occasionally including death. For a bone marrow transplant to be successful, it must be an extremely close match; the best donor is a sibling.
CRISPR
However, there is another possible cure in development thanks to the gene editing technology CRISPR/Cas9. CRISPR may be explained as a segment of DNA which contains short repetitions of base sequences. Some types of bacteria use CRISPR as part of an antiviral system. Cas9 is an enzyme associated with CRISPR, which acts as “molecular scissors”, cutting DNA at a location specified by a guide RNA.
Dr. Emmanuelle Charpentier (one of the founders of CRISPR Therapeutics) and her collaborator Dr. Jennifer Doudna discovered a way to use CRISPR/Cas9 as a gene editing tool, subsequently winning a Nobel Prize in 2020 for their work.
So how does CRISPR/Cas9 gene editing actually work?
This technology allows scientists to edit someone’s genes by precisely cutting DNA and then harnessing natural DNA repair processes to modify the gene. This is accomplished by using a guide RNA (gRNA) to pilot the location at which the Cas9 enzyme will cut the DNA. Gene editing can be achieved by a few different processes: disrupting, deleting, and correcting/inserting.
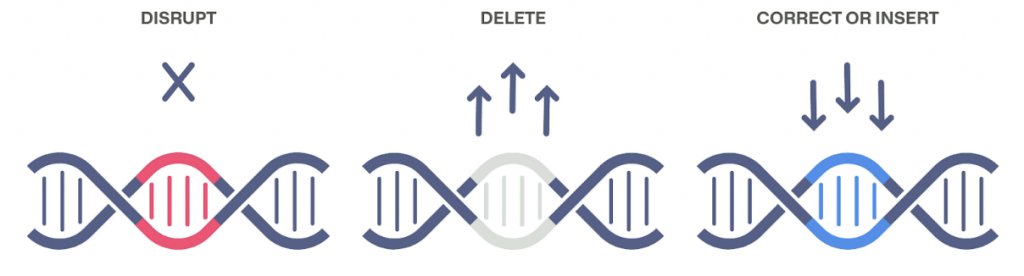
The process of disrupting involves one gRNA making a single cut in the DNA which is then repaired by natural processes. This snip results in either the addition or deletion of base pairs (small sections of coding in your DNA) depending on the intention.
Deleting, when it comes to gene editing, is used when there is a sequence of base pairs intervening with a particular function of a person’s DNA. In this instance, two gRNAs are used to target separate sites for the Cas9 enzyme to cut. After the gene is removed, the separate ends are joined together.
Finally, for correcting or inserting a gene, a genetic template would be added alongside the CRISPR/Cas9 technology, which enables the cell to correct the gene or insert a new one.
In order to administer the treatment, one of two processes will be used; ex vivo or in vivo. For ex vivo, stem cells are collected from the patient for the gene editing procedure. The cells meant to be modified are isolated, then the CRISPR/Cas9 is delivered to those cells. After treatment, the modified cells are tested, and then frozen to be sent to the hospital. The patient will then undergo chemotherapy to make room in their bone marrow for the edited cells to grow and take hold. Ultimately, the modified cells are thawed and infused into the patient where the end goal is for the cells to grow in the bone marrow. In vivo treatment involves packaging the CRISPR/Cas9 in delivery vehicles, such as lipid nanoparticles. These are then inserted into the patient either systematically or directly into the target organ for the desired treatment.
For treating sickle cell disease, an ex vivo treatment is used. The CRISPR/Cas9 cuts DNA in the gene dealing with bone marrow stem cells allowing for a blocked form of hemoglobin, usually only present in the fetus, to be available for use. This gene will instead instruct the hemoglobin in a way that will no longer produce sickle-shaped red blood cells.
Treatment Approval
At this point in time, a treatment called Casgevy, which is derived from CRISPR/Cas9, has been approved to treat sickle cell disease patients in the United Kingdom. The conditions that must be met include the patient being at least 12 years old, and having experienced repeated bouts of extreme pain due to the disease. There is no upper age limit and patients are not excluded if they have extreme organ damage from sickle cell disease. The company is currently testing the treatment for children aged five to eleven.
As for United States approval, the Food and Drug Administration is currently expected to be approving the Casgevy treatment for use around December 8th. Depending on the F.D.A.’s decision, there will likely be similar restrictions around the treatment.
Sickle cell disease is a debilitating genetic condition having over 300 thousand people born with it annually. Millions of people around the world are unable to truly live their lives due to the severe impacts of sickle cell disease, and the CRISPR/Cas9 treatment may be able to change that. This treatment is a revolutionary step towards successfully and effectively treating genetic illnesses, starting with sickle cell, but with the potential to reach even further. In fact, treatment for β-thalassemia, a similar disease to sickle cell, is not far behind.
As of now, CRISPR Therapeutics is working on three other treatments through gene editing. The company has reached the clinical trial stage for their work on immuno-oncology, using cell therapies to treat cancer; and regenerative medicine to replace or repair lost organ or tissue function, using stem cells. Regenerative medicine may also be used to treat diabetes and similar diseases. Additionally CRISPR Therapeutics in the research stage for the use of in vivo gene editing approaches for cardiovascular disease. While it’s too early to know much about the success of any of these, the plausible development of such treatments is a success in and of itself.
The future of medicine, of curing what once seemed incurable, is right around the corner.
Bibliography:
Kolata, Gina. “Panel Says That Innovative Sickle Cell Cure Is Safe Enough for Patients”. New York Times. 31 Oct. 2023, https://www.nytimes.com/2023/10/31/health/sickle-cell-fda-cure-crispr.html?smid=nytcore-ios-share&referringSource=articleShare. Accessed 29 Nov. 2023.
CDC. “What Is Sickle Cell Disease?” Centers for Disease Control and Prevention, 18 Aug. 2022, www.cdc.gov/ncbddd/sicklecell/facts.html. Accessed 25 Nov. 2023.
Zakrzewski, Wojciech, et al. “Stem Cells: Past, Present, and Future.” Stem Cell Research & Therapy, vol. 10, no. 1, BioMed Central, Feb. 2019, https://doi.org/10.1186/s13287-019-1165-5. Accessed 24 Nov. 2023.
Kolata, Gina. “Sickle-Cell Treatment Created with Gene Editing Wins U.K. Approval.” New York Times, Published 16 Nov. 2023, Updated 20 Nov. 2023, www.nytimes.com/2023/11/16/health/casgevy-sickle-cell-crispr.html. Accessed 29 Nov. 2023.
“Gene Editing.” CRISPR Therapeutics, 2023, https://crisprtx.com/gene-editing Accessed 24 Nov. 2023.
“Hemoglobinopathies.” CRISPR Therapeutics, 2023, https://crisprtx.com/focus-areas/hemoglobinopathies. Accessed 29 Nov. 2023.
“Current Clinical Research.” CRISPR Therapeutics, 2023, https://crisprtx.com/patients/current-clinical-research. Accessed 29 Nov. 2023.
“Get the Facts on Gene Editing.” CRISPR Therapeutics, Dec. 2020, https://s3.us-east-1.amazonaws.com/crisprtx-assets.investeddigital.com/Get_the_Facts_on_Gene_Editing_Resource.pdf. Accessed 29 Nov. 2023.
“Get the Facts on Hemoglobinopathies: Sickle Cell Disease (SCD) and β-thalassemia.” CRISPR Therapeutics, Feb. 2021, https://s3.us-east-1.amazonaws.com/crisprtx-assets.investeddigital.com/Get_the_Facts_on_Hem_Resource.pdf. Accessed 29 Nov. 2023.
“Pipeline.” CRISPR Therapeutics, 2023, https://crisprtx.com/pipeline. Accessed 29 Nov. 2023.
“Immuno-Oncology.” CRISPR Therapeutics, 2023, https://crisprtx.com/focus-areas/immuno-oncology. Accessed 29 Nov. 2023.
“In Vivo.” CRISPR Therapeutics, 2023, https://crisprtx.com/focus-areas/in-vivo. Accessed 29 Nov. 2023.
“Regenerative Medicine.” CRISPR Therapeutics, 2023, https://crisprtx.com/focus-areas/regenerative-medicine. Accessed 29 Nov. 2023.
“Vertex and CRISPR Therapeutics Announce Authorization of the First….” CRISPR Therapeutics, 16 Nov. 2023, https://crisprtx.com/about-us/press-releases-and-presentations/vertex-and-crispr-therapeutics-announce-authorization-of-the-first-crispr-cas9-gene-edited-therapy-casgevy-exagamglogene-autotemcel-by-the-united-kingdom-mhra-for-the-treatment-of-sickle-cell-disease-and-transfusi. Accessed 29 Nov. 2023.
Lanzkron, Sophie et al. “Mortality rates and age at death from sickle cell disease: U.S., 1979-2005.” Public health reports (Washington, D.C. : 1974) vol. 128,2 (2013): 110-6. doi:10.1177/003335491312800206, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3560868/. Accessed 30 Nov. 2023